ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Descrição

Disease Control Data, Ankylosing Spondylitis
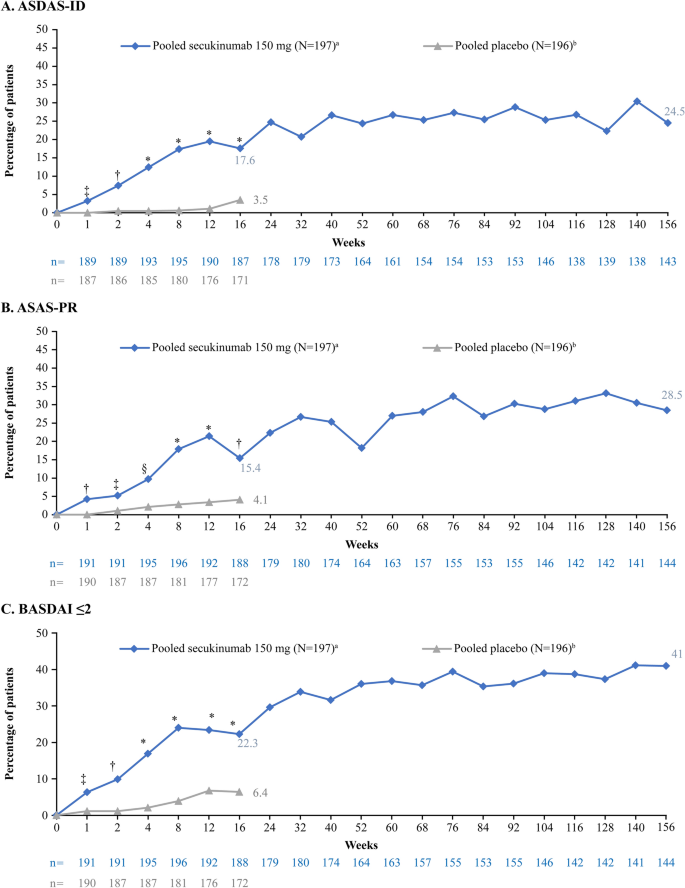
Achievement of Remission Endpoints with Secukinumab Over 3 Years in Active Ankylosing Spondylitis: Pooled Analysis of Two Phase 3 Studies

Oral Abstracts - 2020 - International Journal of Rheumatic Diseases - Wiley Online Library

Long-Term Safety and Efficacy of Ixekizumab in Patients With Axial Spondyloarthritis: 3-year Data From the COAST Program
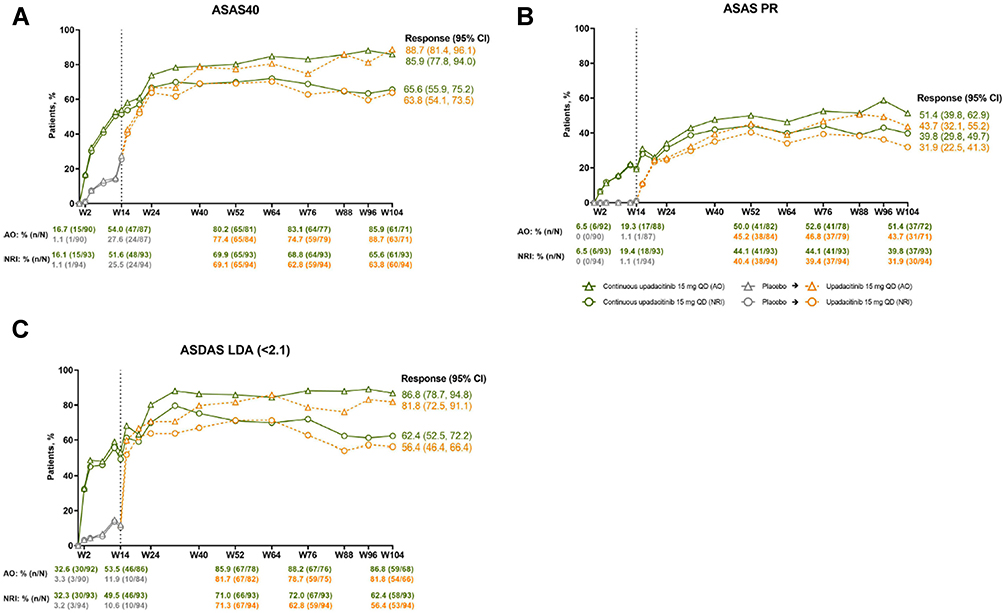
Management of axial spondyloarthritis
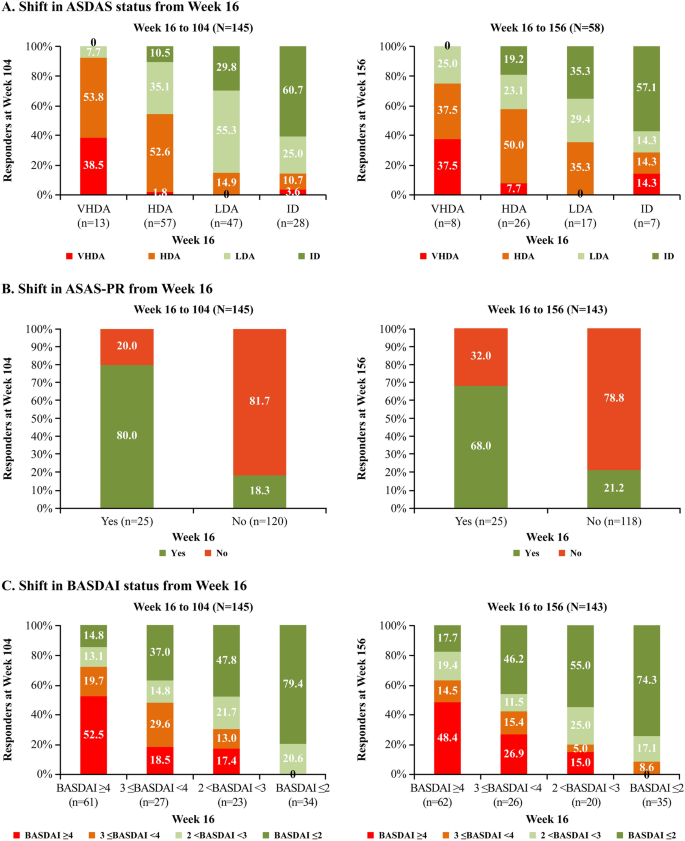
Achievement of Remission Endpoints with Secukinumab Over 3 Years in Active Ankylosing Spondylitis: Pooled Analysis of Two Phase 3 Studies
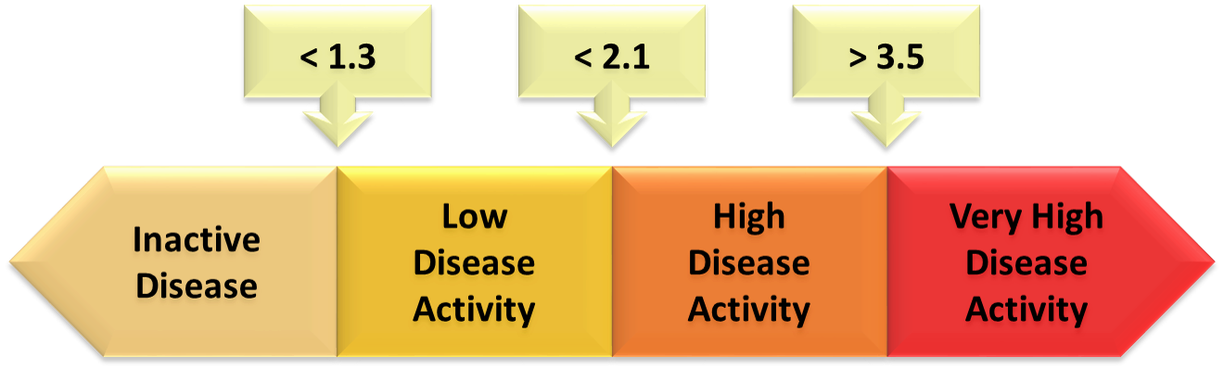
ASDAS calculator - ASAS

The Sensitivity to Change of the ASAS Health Index in an Observational Real-Life Cohort Study

Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial

Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study
de
por adulto (o preço varia de acordo com o tamanho do grupo)







