Statistics in Medicine — Reporting of Subgroup Analyses in
Por um escritor misterioso
Descrição
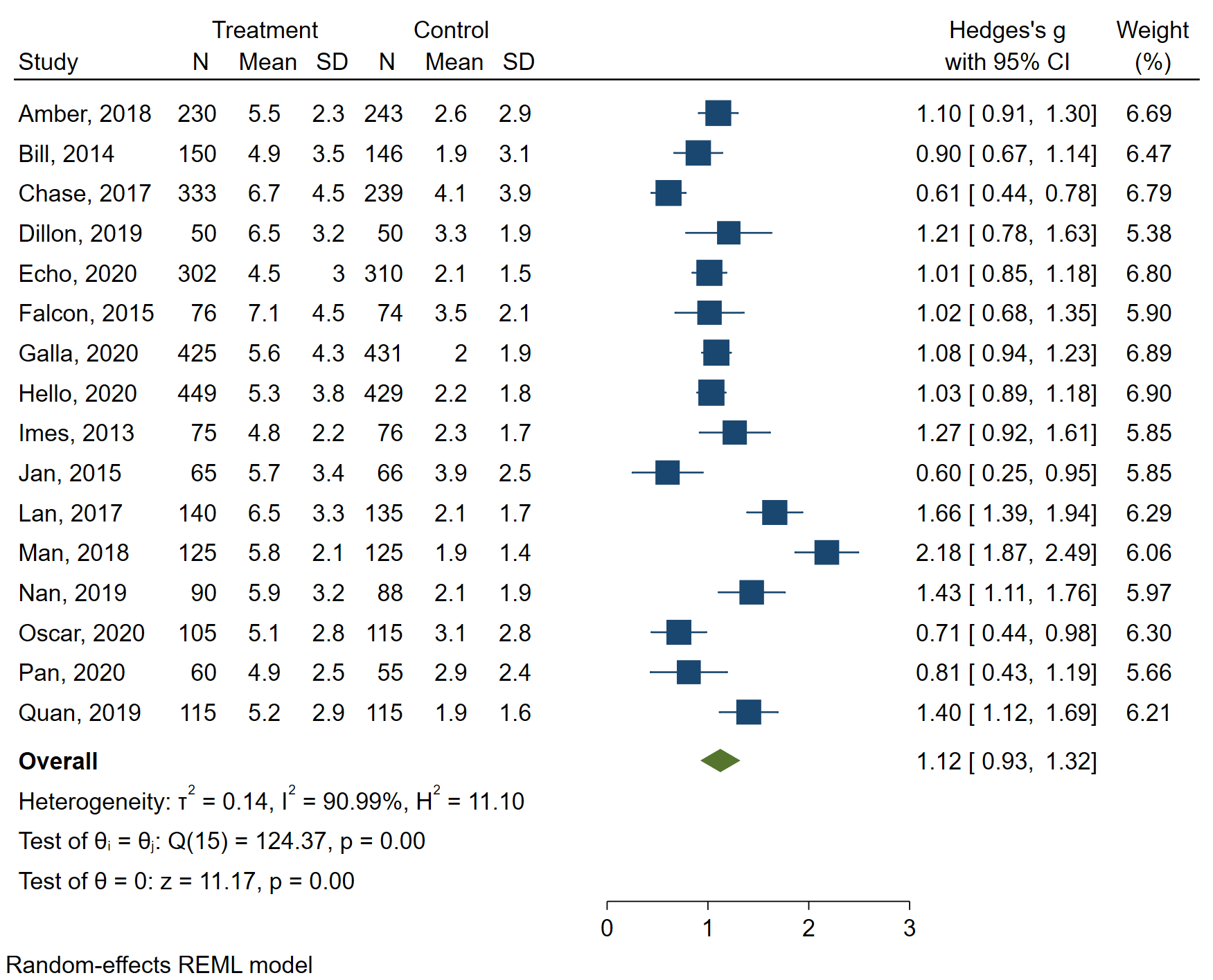
Introduction to Meta-Analysis in Stata

The influence of study characteristics on reporting of subgroup analyses in randomised controlled trials: systematic review

Cumulative subgroup analysis to reduce waste in clinical research for individualised medicine, BMC Medicine

Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis - The Lancet

Completeness of reporting and risks of overstating impact in cluster randomised trials: a systematic review - The Lancet Global Health
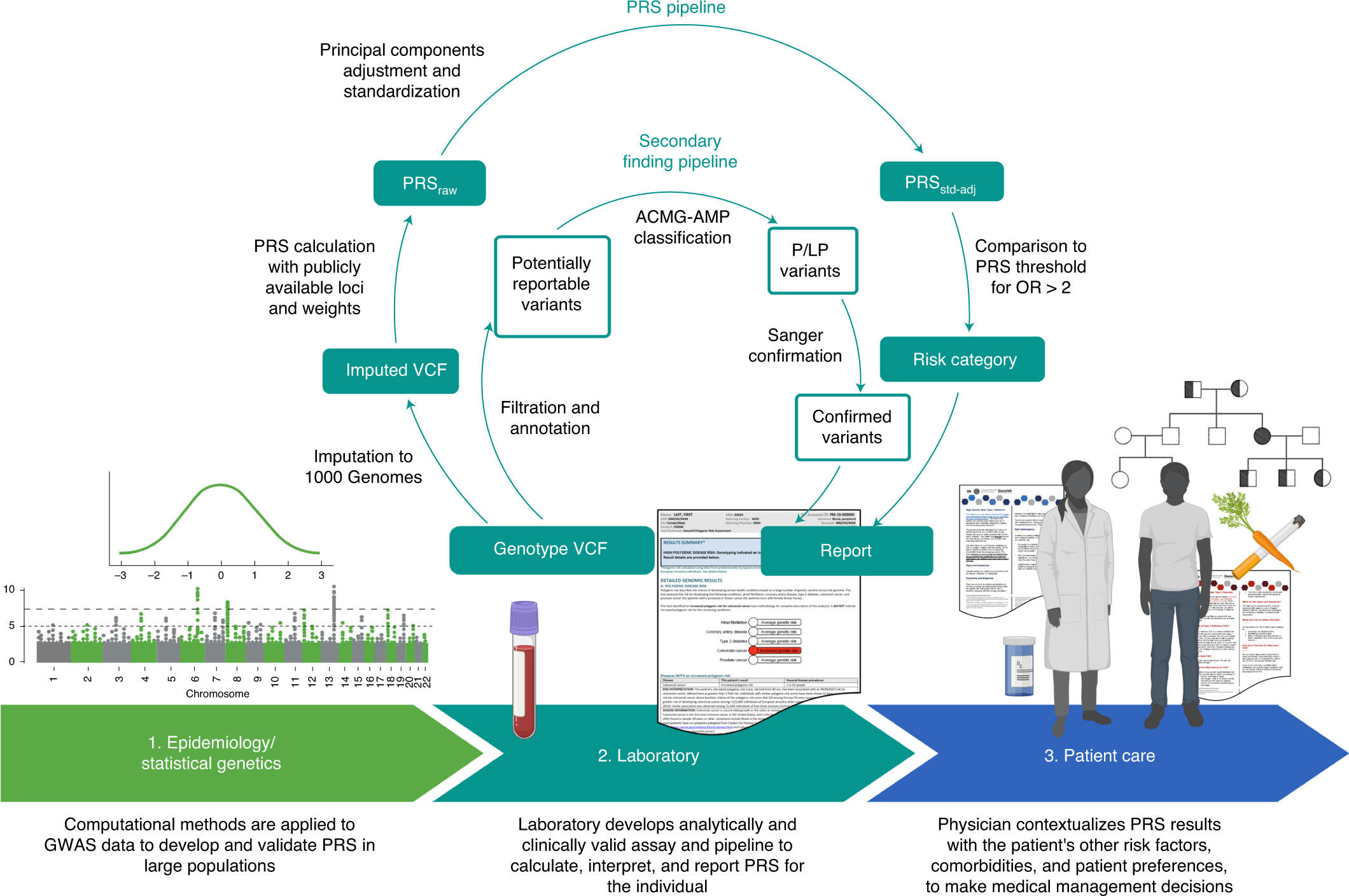
Development of a clinical polygenic risk score assay and reporting workflow

A comprehensive analysis of the efficacy and safety of COVID-19 vaccines: Molecular Therapy

Subgroup Analyses: Subpar or Sublime?

Biostatistics Primer: What a Clinician Ought to Know: Subgroup Analyses - ScienceDirect
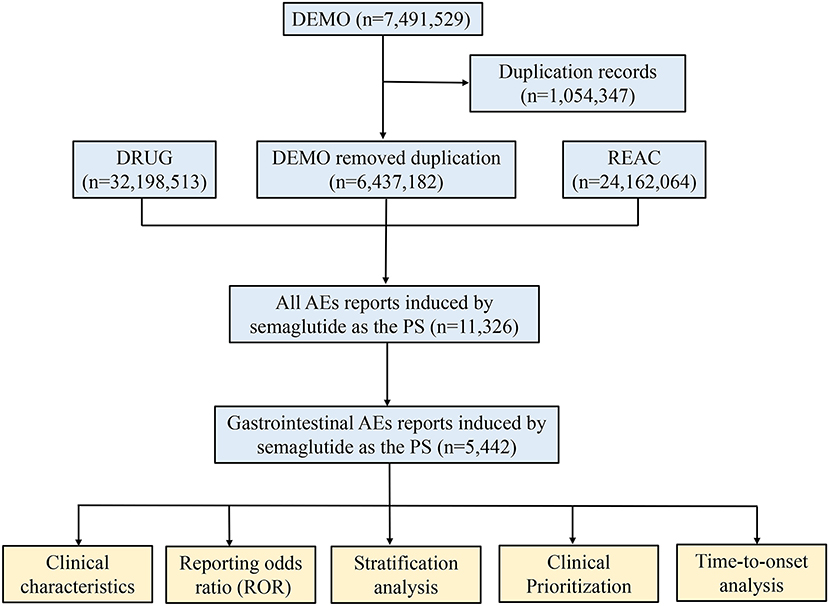
Frontiers Gastrointestinal adverse events associated with semaglutide: A pharmacovigilance study based on FDA adverse event reporting system
de
por adulto (o preço varia de acordo com o tamanho do grupo)