FDA OKs Cell Therapy to Lower Infection Risk After Stem Cell Transplant
Por um escritor misterioso
Descrição
Omidubicel reduced infections in blood cancer patients from 60% to 39% at 100 days posttransplant
PDF) Nonmyeloablative Allogeneic Hematopoietic Stem Cell
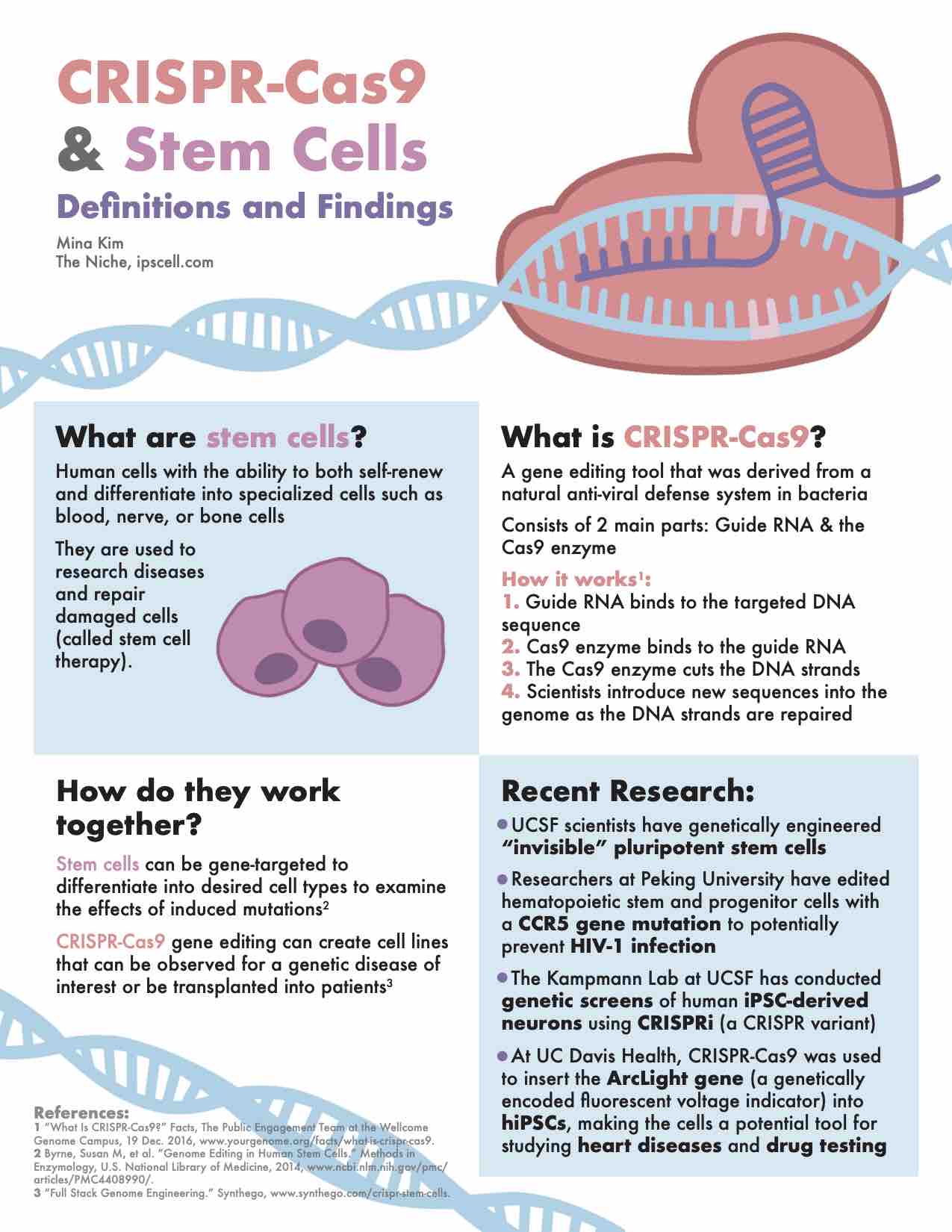
New research on CRISPR gene-editing in stem cells, infographic

Review of 4 cell therapy types under study for COVID-19 - The Niche
FDA investigates risk of secondary malignancies with CAR T-cell
:max_bytes(150000):strip_icc()/Health-Stocksy-3418126-80bec79dd1a145419ff8a990f1b32b57.jpg)
FDA Approves Fecal Transplant Therapy for Recurrent C. Diff

Critical review on the physical and mechanical factors involved in
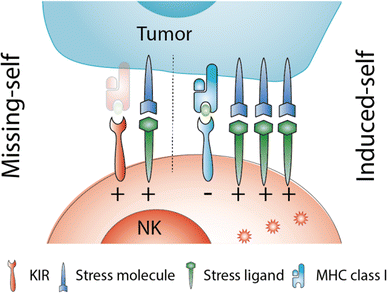
NK cell therapy after hematopoietic stem cell transplantation: can

New cell therapy approaches yield fewer complications after organ

Adult Acute Lymphoblastic Leukemia Treatment (PDQ®) - PDQ Cancer
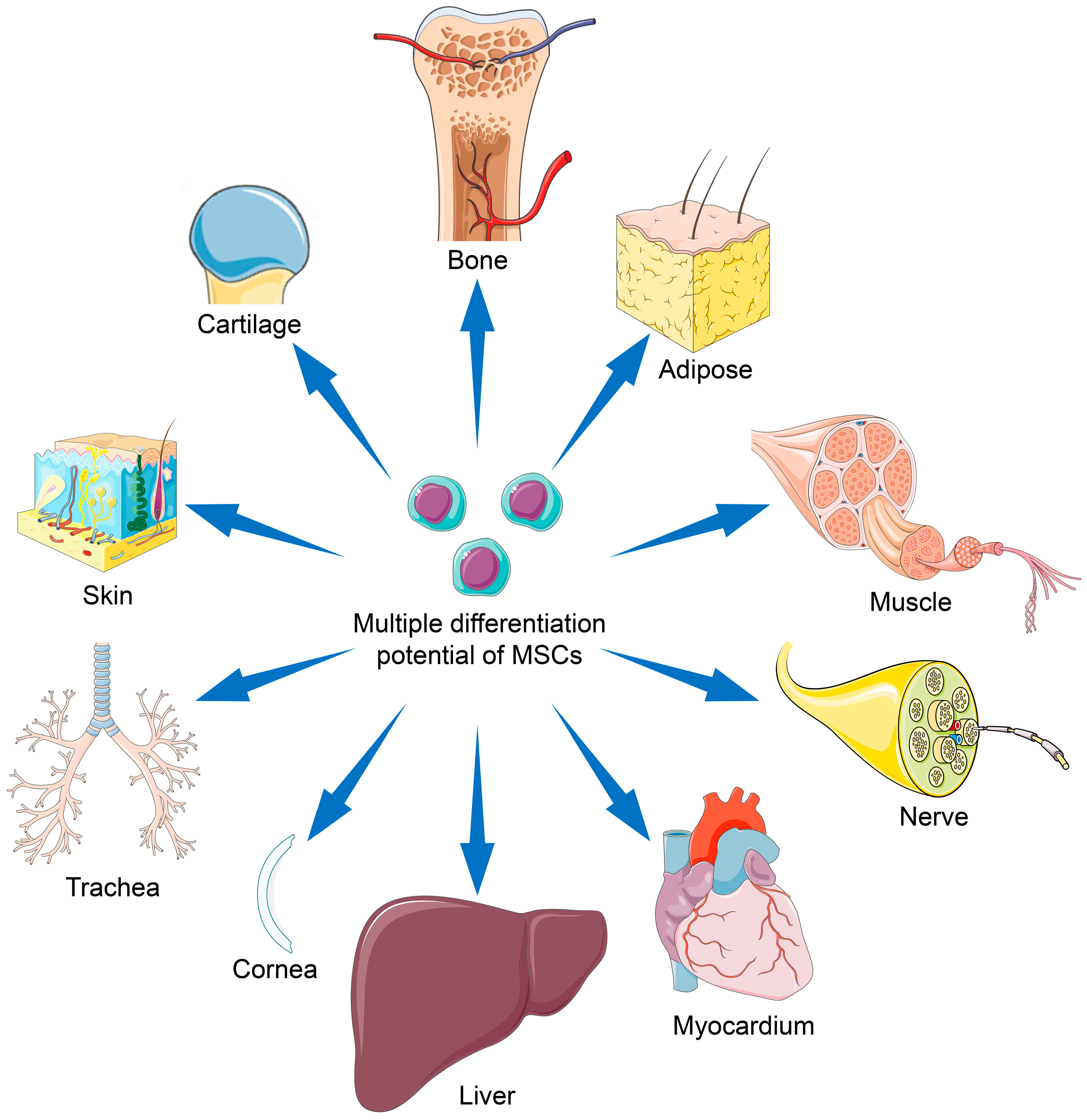
Cells, Free Full-Text

FDA Approves First CRISPR/Cas9 Gene-Editing Therapy
de
por adulto (o preço varia de acordo com o tamanho do grupo)







