18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial - The Lancet
Por um escritor misterioso
Descrição
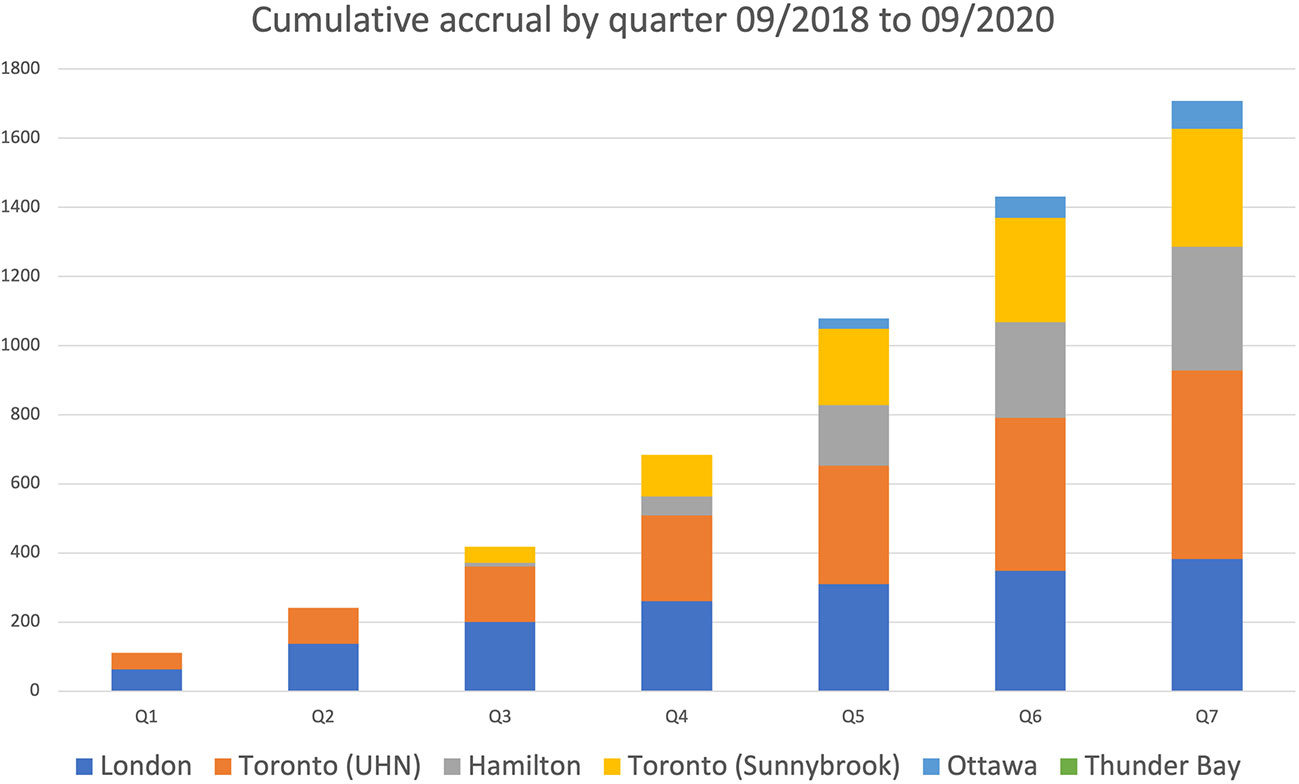
Frontiers Establishing a Provincial Registry for Recurrent Prostate Cancer: Providing Access to PSMA PET/CT in Ontario, Canada

Diagnostic Performance and Safety of 18F-rhPSMA-7.3 Positron Emission Tomography in Men With Suspected Prostate Cancer Recurrence: Results From a Phase 3, Prospective, Multicenter Study (SPOTLIGHT)

Differences in Failure-Free Survival After Salvage Radiotherapy Guided by Conventional Imaging Versus 18F-Fluciclovine PET/CT in Postprostatectomy Patients: A Post Hoc Substratification Analysis of the EMPIRE-1 Trial
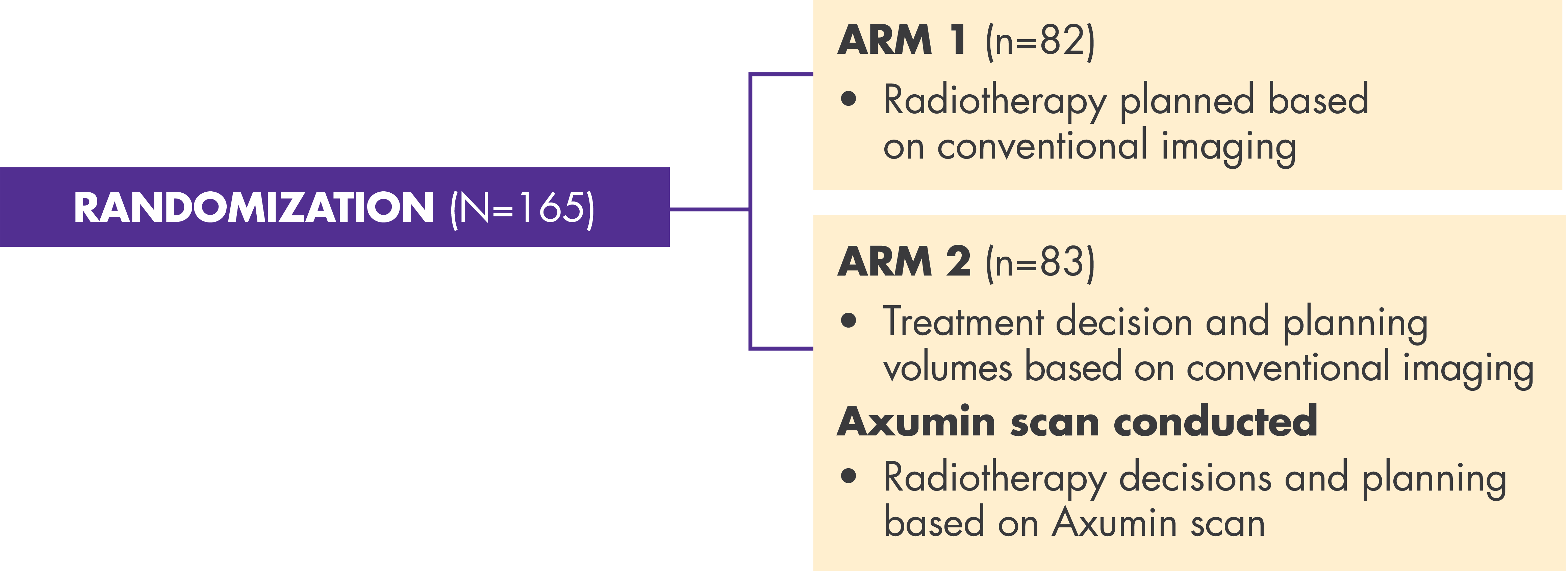
Axumin LOCATE Trial Results Axumin® (fluciclovine F 18) Injection
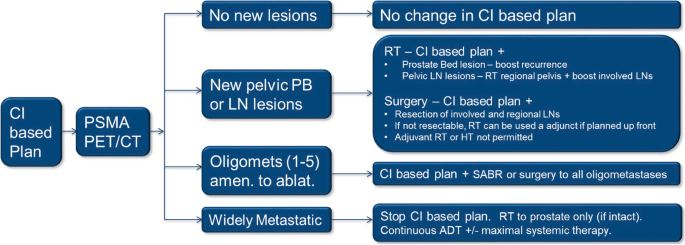
PSMA PET/CT guided intensification of therapy in patients at risk of advanced prostate cancer (PATRON): a pragmatic phase III randomized controlled trial, BMC Cancer

18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial - The Lancet

O papel do PET/CT com 18F-fluciclovina no prognóstico de pacientes com câncer de próstata - Oncologia Brasil

Improvements in Prostate Cancer Management: Focus on Imaging and Treatment - European Medical Journal

Appropriate Use Criteria for Prostate-Specific Membrane Antigen PET Imaging
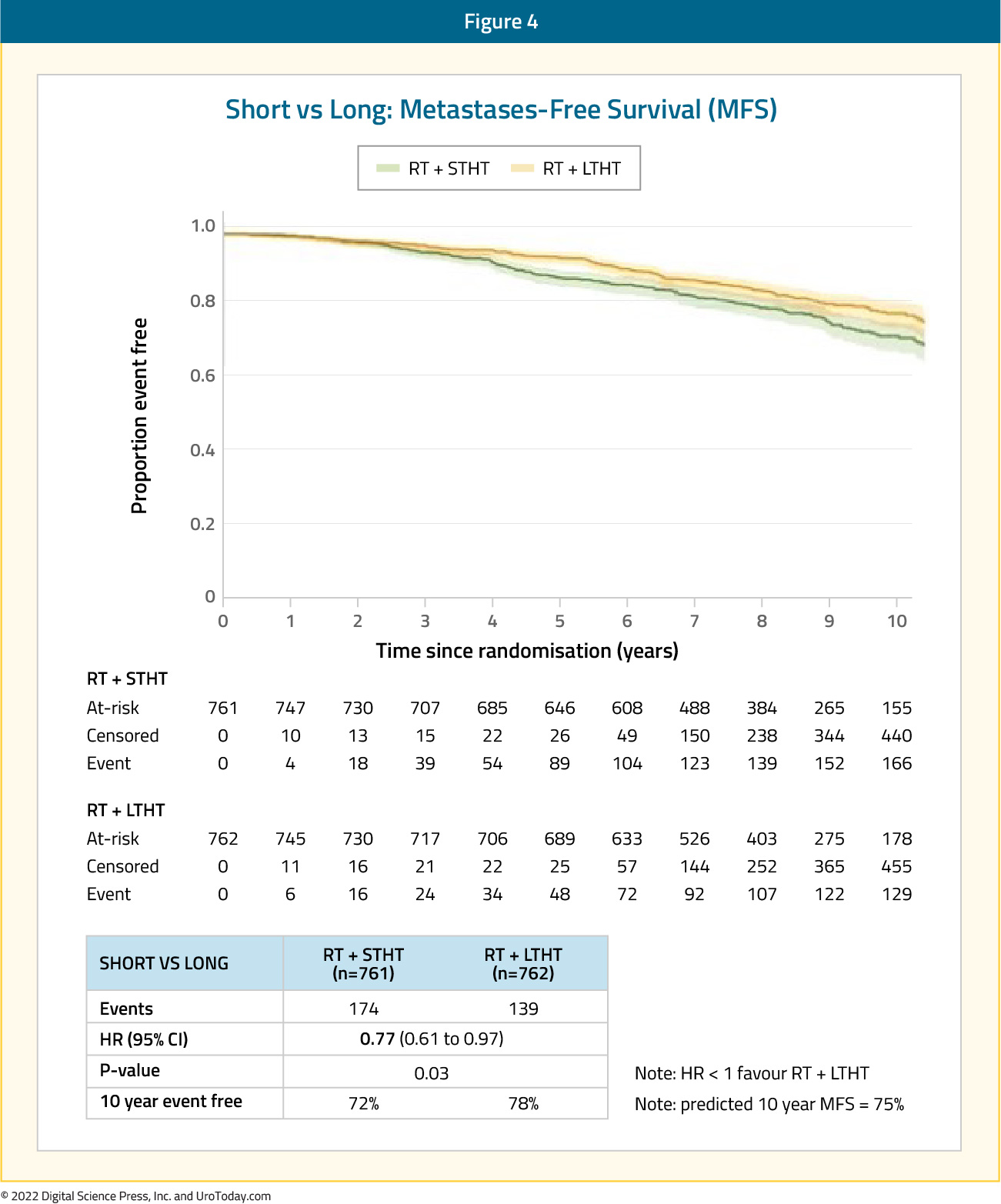
Radiotherapy in Prostate Cancer: Utilization of Adjuvant and Salvage Radiotherapy
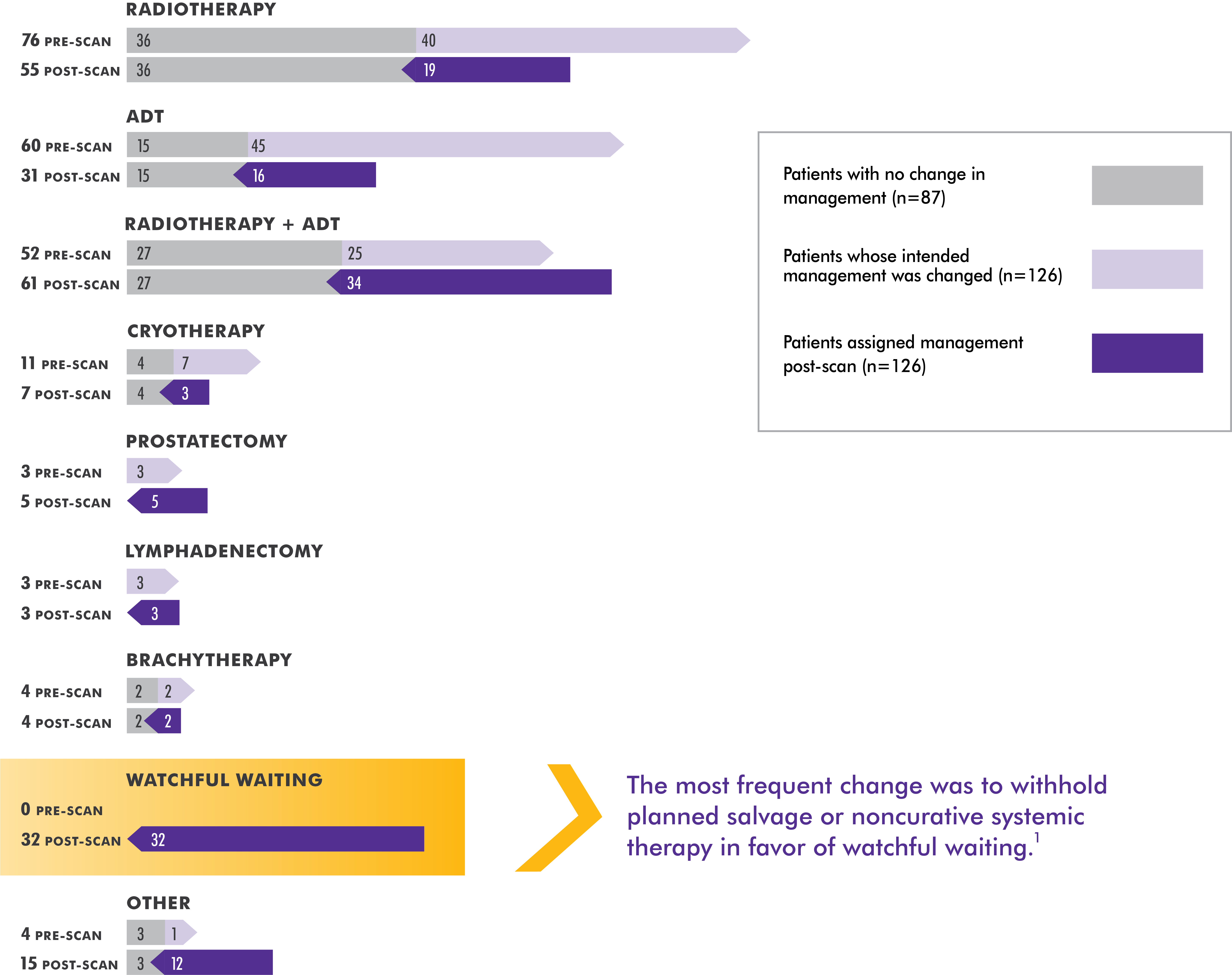
Axumin LOCATE Trial Results Axumin® (fluciclovine F 18) Injection

Effect of 18F-DCFPyL PET/CT on the Management of Patients with Recurrent Prostate Cancer: Results of a Prospective Multicenter Registry Trial
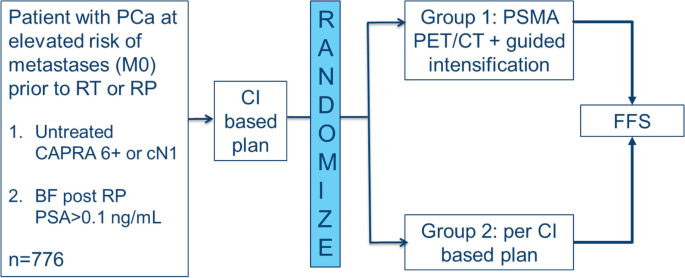
PSMA PET/CT guided intensification of therapy in patients at risk of advanced prostate cancer (PATRON): a pragmatic phase III randomized controlled trial, BMC Cancer
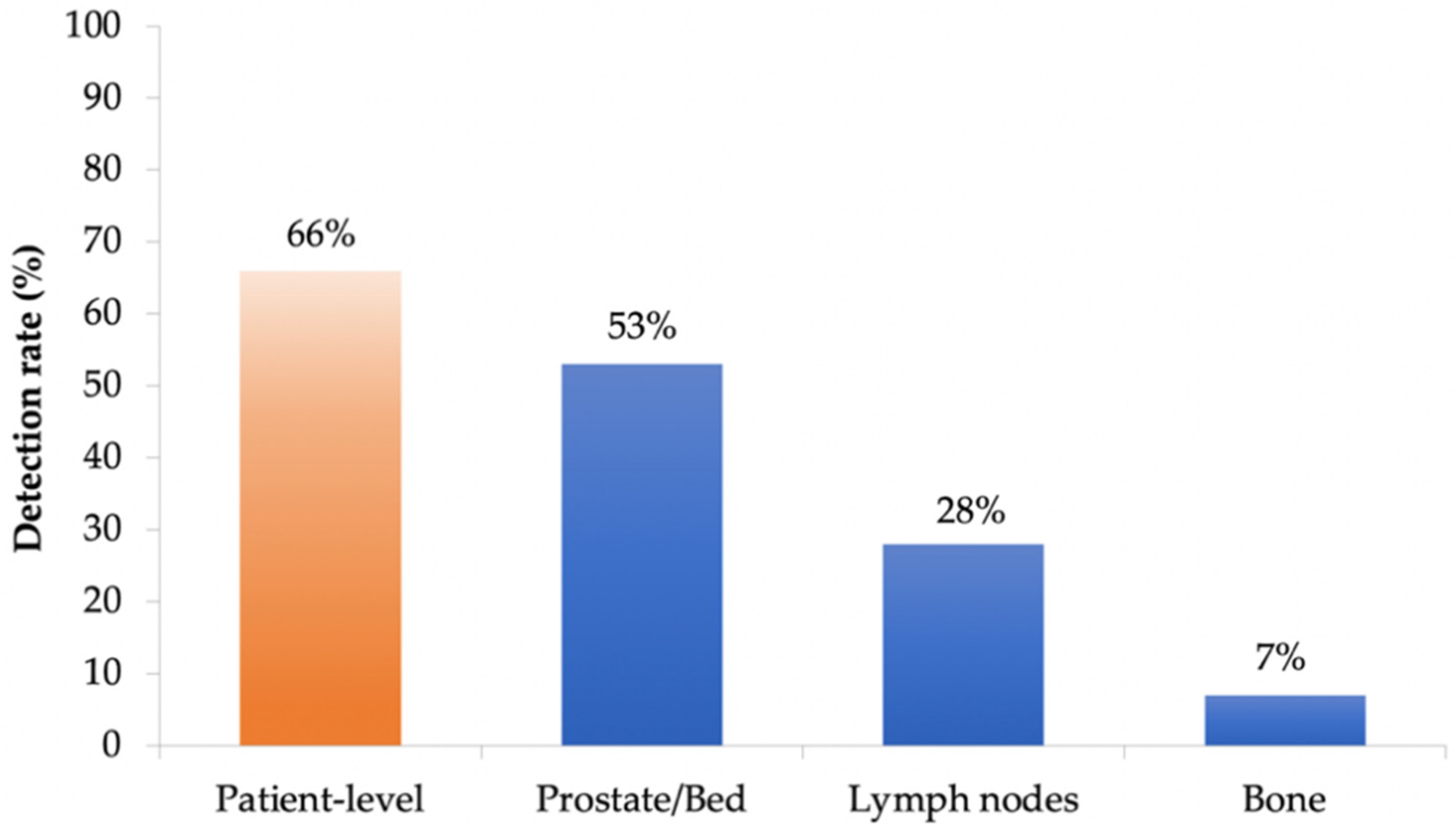
Cancers, Free Full-Text
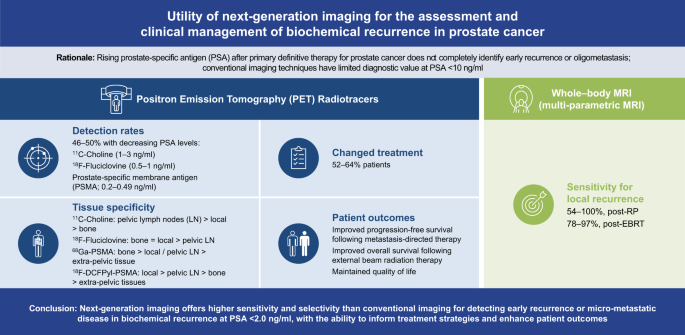
Application of next-generation imaging in biochemically recurrent prostate cancer
de
por adulto (o preço varia de acordo com o tamanho do grupo)






